Pipeline by indication
NETRIS Pharma is conducting a large Clinical Development Plan with its lead asset NP137. A pipeline of new products targeting other ligands is also in construction.

Pipeline
| Program Target | Indication | Discovery | IND Enabling | Phase I | Phase II | Late-Stage Development |
|---|---|---|---|---|---|---|
|
NP137 + Pembro(*)/Carbotaxol
Netrin-1
|
Endometrium / Cervix |
|||||
|
NP137 + Approved Immune Checkpoint
Netrin-1
|
Basket: • NSLCC |
|||||
|
NP137 + Atezo-Bev
Netrin-1
|
Liver |
|||||
|
NP137 + Folfirinox
Netrin-1
|
Pancreas – Locally advanced |
|||||
|
NP137 + Folfirinox
Netrin-1
|
Pancreas – Resectable |
|||||
|
NP137P
Netrin-1
|
Endometriosis |
|||||
|
NP137R
Netrin-1
|
Netrin-1 expressing cancer (radiotherapy) |
|||||
|
NP800
Undisclosed target
|
Glioblastoma |
Clinical Development
NETRIS Pharma's clinical development plan encompasses five recruiting clinical trials aimed at confirming the ability of its lead asset, NP137, to reduce tumors growth and aggressiveness as well as to overcome resistance to current standard of care. Through its long-term collaboration with the Leon Bérard Comprehensive Cancer Center, NETRIS Pharma accesses a unique set of multidisciplinary technological platforms to conduct ambitious ancillary research programs to identify relevant biomarkers and better characterize patients who shall most likely benefit from the treatment.

Endometriosis
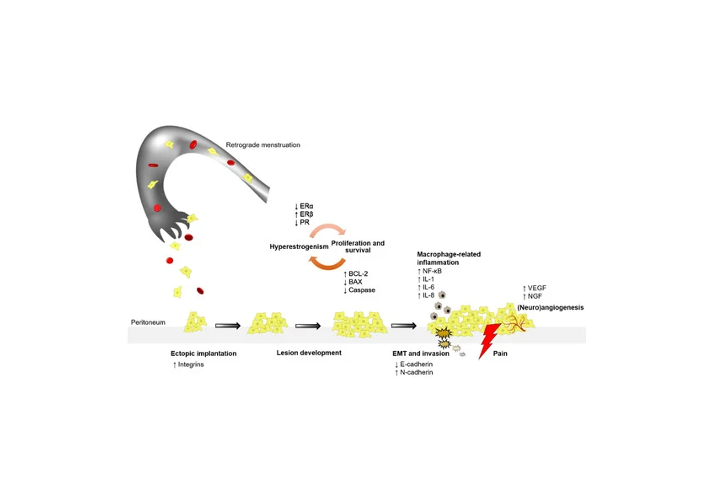
Endometriosis is a disabling disease affecting nearly one in ten women characterized by the uncontrolled development of endometrium tissues outside the uterus. The disease is associated with chronic pain and fertility issues. Available therapeutic options are limited to symptomatic treatment to alleviate pain or surgery, urging health and political authorities to declare endometriosis as “National Health Cause” such as in France in 2022
A number of publications showed the expression of netrin-1 in endometriosis lesions and the role of the Epithelial-to-Mesenchymal Transition (EMT) appears to be an important factor event in the progression of the disease.

Based on this strong scientific rationale and given the lack of therapeutic options for women suffering from this debilitating disease, NETRIS Pharma decided to launch a development program aimed at delivering the first non-hormonal disease modifying treatment in endometriosis with a monoclonal antibody developed by NETRIS Pharma targeting netrin-1.
This project is supported by a 3.9 M€ grant from BPI.

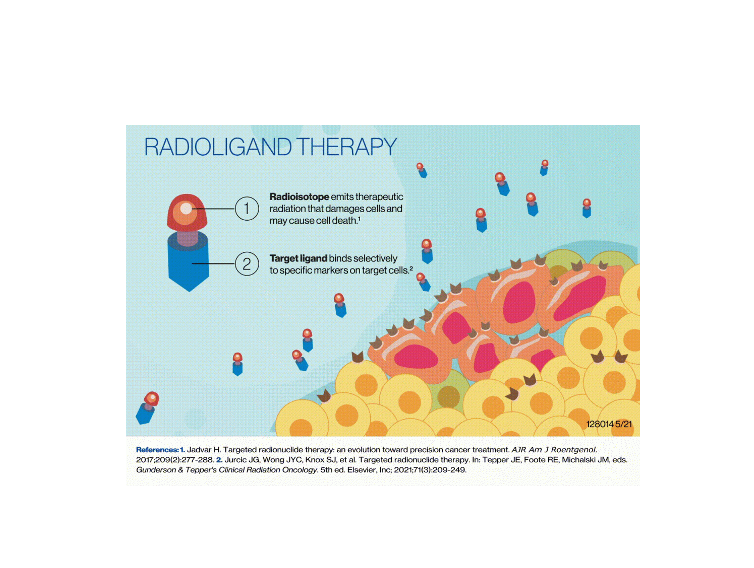
Anti-netrin1 RadioLigand Therapy
Radioligand Therapy consists in the use of radioisotopes coupled to targeting moieties such as peptide or antibodies to achieve a localized delivery of radiotherapy for diagnostic and therapeutic purposes.
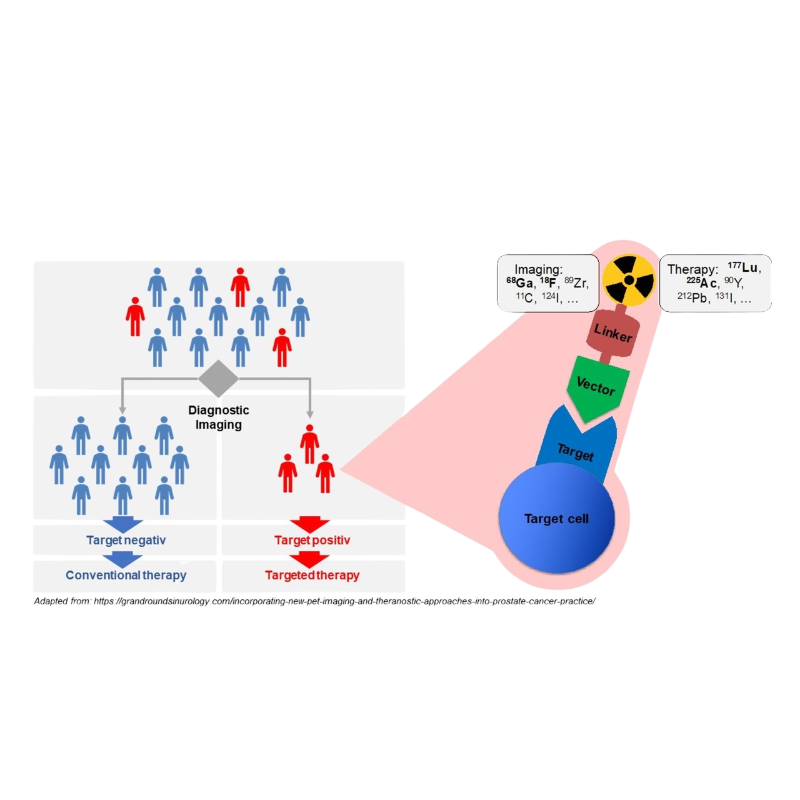

The confined expression of netrin-1 in the tumor microenvironment sparked interest in developing a novel radio-conjugate approach, coupling anti-netrin1 compounds with selected radioisotopes, to more selectively target tumor cells. Pharmacological models confirm the potential of this innovative method for both imaging and therapeutic purposes.
This project is conducted in collaboration with INSERM and Orano.